EXPAREL® (bupivacaine liposome injectable suspension)
EXPAREL is a local anesthetic administered at the time of surgery to control pain and reduce or eliminate the use of opioids for acute postsurgical pain.
EXPAREL is the only non-opioid option approved to produce postsurgical local analgesia via infiltration in patients aged 6 years and older and regional analgesia in adults via an interscalene brachial plexus nerve block, sciatic nerve block in the popliteal fossa, and an adductor canal block.*
When injected into the surgical site, EXPAREL turns off the body’s pain signals, numbing the area where surgery has occurred for the first few days following the procedure. To date, EXPAREL has been used in more than 15 million patients.
*Safety and efficacy have not been established in other nerve blocks.
What makes EXPAREL different?
EXPAREL delivers targeted pain relief directly at the surgical site, unlike opioids, which work systemically and affect the entire body.
- Requires only one dose, administered by a doctor during surgery.
- Provides pain control for the first few days after surgery, when patients need it most.
- Proven in clinical trials to reduce the need for opioids in both adults and children.*
- Patients went longer before needing opioids compared to those who didn’t receive it.
- Patients required fewer opioids compared to those who didn’t receive it.
*The clinical benefit of the decrease in opioid consumption was not demonstrated in the pivotal trials.
How it works
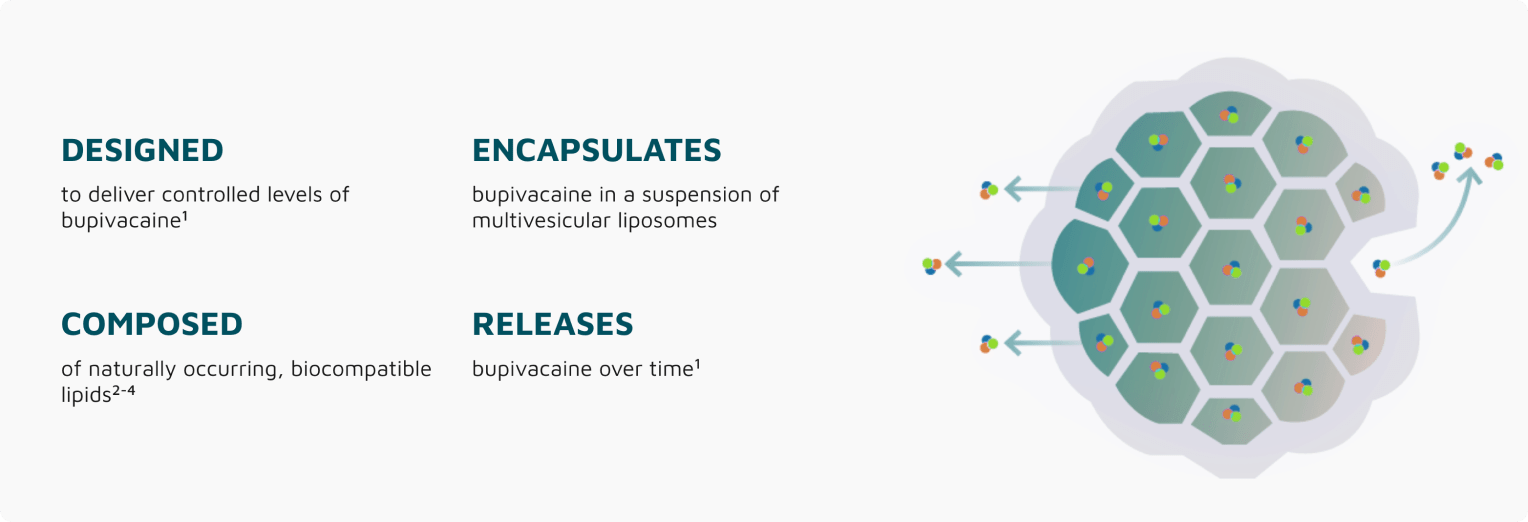
Releases Bupivacaine Over Time
EXPAREL consists of bupivacaine, a local anesthetic, encapsulated in our proprietary multivesicular liposome drug delivery platform.
Applications
EXPAREL can be used in a wide range of procedures across multiple specialties.
- General
Bariatric, Colon, Hemorrhoid, Hernia, Kidney, Stomach
- Oral and maxillofacial
Implants, TMJ, Wisdom Teeth Extraction
- Orthopedic
Ankle, Foot, Hand, Hip, Knee, Shoulder, Spine
- Pediatric
ACL, Scoliosis/Spine, Tonsillectomy, Wisdom Teeth
- Women’s health
Breast, Cesarean Delivery (C-Section), Fibroid, Hysterectomy
Testimonials

After experiencing a painful C-section recovery, Meredith shares how choosing a non-opioid path the second time changed everything.

After multiple knee surgeries and years of pain, former NFL player Brandon Chubb shares how a non-opioid approach brought real relief—and got him back to life off the sidelines.

From treating elite athletes to navigating her own recovery, Dr. West explains why non-opioid options are changing the game in surgical care and recovery.

Lea, a college soccer player, shares her journey through ACL surgery and explains how a non-opioid pain management approach helped her get back on the field sooner.

Dr. Paul Sethi, an orthopedic surgeon specializing in sports medicine, discusses how a non-opioid pain management approach can help get patients–especially young athletes–back on the field.
Indications
EXPAREL® (bupivacaine liposome injectable suspension) is indicated to produce postsurgical local analgesia via infiltration in patients aged 6 years and older and regional analgesia in adults via an interscalene brachial plexus nerve block, sciatic nerve block in the popliteal fossa, and an adductor canal block. Safety and efficacy have not been established in other nerve blocks.
Important safety information
- EXPAREL is contraindicated in obstetrical paracervical block anesthesia.
- Adverse reactions reported in adults with an incidence greater than or equal to 10% following EXPAREL administration via infiltration were nausea, constipation, and vomiting; adverse reactions reported in adults with an incidence greater than or equal to 10% following EXPAREL administration via nerve block were nausea, pyrexia, headache, and constipation.
- Adverse reactions with an incidence greater than or equal to 10% following EXPAREL administration via infiltration in pediatric patients six to less than 17 years of age were nausea, vomiting, constipation, hypotension, anemia, muscle twitching, vision blurred, pruritus, and tachycardia.
- Do not admix lidocaine or other non-bupivacaine local anesthetics with EXPAREL. EXPAREL may be administered at least 20 minutes or more following local administration of lidocaine.
- EXPAREL is not recommended to be used in the following patient populations: patients <6 years old for infiltration, patients younger than 18 years old for nerve blocks, and/or pregnant patients.
- Because amide-type local anesthetics, such as bupivacaine, are metabolized by the liver, EXPAREL should be used cautiously in patients with hepatic disease.
Warnings and precautions specific to EXPAREL
- Avoid additional use of local anesthetics within 96 hours following administration of EXPAREL.
- EXPAREL is not recommended for the following types or routes of administration: epidural, intrathecal, regional nerve blocks other than interscalene brachial plexus nerve block, sciatic nerve block in the popliteal fossa, and adductor canal block, or intravascular or intra-articular use.
- The potential sensory and/or motor loss with EXPAREL is temporary and varies in degree and duration depending on the site of injection and dosage administered and may last for up to 5 days, as seen in clinical trials.
Warnings and precautions for bupivacaine-containing products
- Central Nervous System (CNS) Reactions: There have been reports of adverse neurologic reactions with the use of local anesthetics. These include persistent anesthesia and paresthesia. CNS reactions are characterized by excitation and/or depression.
- Cardiovascular System Reactions: Toxic blood concentrations depress cardiac conductivity and excitability, which may lead to dysrhythmias, sometimes leading to death.
- Allergic Reactions: Allergic-type reactions (eg, anaphylaxis and angioedema) are rare and may occur as a result of hypersensitivity to the local anesthetic or to other formulation ingredients.
- Chondrolysis: There have been reports of chondrolysis (mostly in the shoulder joint) following intra-articular infusion of local anesthetics, which is an unapproved use.
- Methemoglobinemia: Cases of methemoglobinemia have been reported with local anesthetic use.
References
- Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
- Angst MS, Drover DR. Pharmacology of drugs formulated with DepoFoam: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin Pharmacokinet. 2006;45(12):1153-1176.
- Kohn FR, Malkmus SA, Brownson EA, Rossi SS, Yaksh TL. Fate of the predominant phospholipid component of DepoFoam drug delivery matrix after intrathecal administration of sustained-release encapsulated cytarabine in rats. Drug Deliv. 1998;5(2):143-151.
- Richard BM, Newton P, Ott LR, et al. The safety of EXPAREL® (bupivacaine liposome injectable suspension) administered by peripheral nerve block in rabbits and dogs. J Drug Deliv. 2012;2012:962101.